Research Insight
Genetic Approaches and Breeding Strategies for Enhancing Northern Corn Leaf Blight Resistance in Maize 
 Author
Author  Correspondence author
Correspondence author
Maize Genomics and Genetics, 2024, Vol. 15, No. 6 doi: 10.5376/mgg.2024.15.0027
Received: 27 Sep., 2024 Accepted: 31 Oct., 2024 Published: 16 Nov., 2024
Zhao L., Wu Y.F., Yu X.Q., and Shi J., 2024, Genetic approaches and breeding strategies for enhancing northern corn leaf blight resistance in maize, Maize Genomics and Genetics, 15(6): 282-292 (doi: 10.5376/mgg.2024.15.0027)
This study explores genetic approaches and breeding strategies to enhance maize resistance to northern corn leaf blight (NCLB), focusing on understanding the genetic basis of resistance and identifying key resistance genes and quantitative trait loci (QTL). The findings indicate that effective NCLB resistance is achieved through both qualitative resistance, primarily controlled by major genes such as Ht1, Ht2, Ht3, and Htn1, and quantitative resistance involving multiple genes. Advanced tools, such as genome-wide association studies (GWAS) and QTL mapping, have enabled precise identification and utilization of resistance genes. Biotechnological innovations, including CRISPR/Cas9 gene editing and RNA interference (RNAi) technology, offer targeted opportunities for resistance enhancement. These integrated strategies have successfully developed maize varieties with improved disease resistance and productivity. This study aims to provide a scientific basis for further genetic improvement and insights into sustainable NCLB management strategies in maize breeding.
1 Introduction
Northern corn leaf blight (NCLB), caused by the fungal pathogen Exserohilum turcicum, is a significant foliar disease affecting maize (Zea mays) worldwide (Pan et al., 2022). This disease is responsible for substantial yield losses, particularly in regions with conducive environmental conditions for the pathogen’s proliferation (Rashid et al., 2020; Yang et al., 2021; Thatcher et al., 2022). The pathogen’s ability to infect maize leaves leads to the formation of large, necrotic lesions, which can coalesce and cause extensive leaf damage, ultimately reducing photosynthetic capacity and grain yield (Welz and Geiger, 2000; Ranganatha et al., 2021). The economic impact of NCLB is profound, necessitating effective management strategies to mitigate its effects on maize production (Hurni et al., 2015).
Developing and deploying NCLB-resistant maize varieties is crucial for sustainable maize production. Resistance to NCLB can be conferred by both qualitative and quantitative resistance genes. Qualitative resistance, often governed by single genes such as Ht1, Ht2, Ht3, and Htn1, provides high levels of resistance but can be overcome by new pathogen races (Yang et al., 2021; Thatcher et al., 2022). On the other hand, quantitative resistance, controlled by multiple genes, offers more durable resistance across different environments and pathogen populations (Welz and Geiger, 2000; Ranganatha et al., 2021). The integration of both types of resistance through breeding programs can enhance the resilience of maize crops against NCLB, ensuring stable yields and reducing the reliance on chemical fungicides (Hurni et al., 2015; Rashid et al., 2020).
In this study, we explored the genetic basis of maize resistance to northern corn leaf blight (NCLB) and formulated effective breeding strategies to enhance maize resistance to the disease, in order to provide theoretical support and technical basis for maize NCLB resistance breeding. It is expected to make breakthroughs in improving maize yield and disease resistance.
2 Etiology, Symptoms and Effects of Northern Corn Leaf Blight
2.1 Pathogen and disease cycle
Northern corn leaf blight (NCLB) is caused by the fungal pathogen Setosphaeria turcica (Anamorph exserohilum turcicum), which is a significant threat to maize production worldwide (Welz and Geiger, 2000; Yang et al., 2021). The pathogen exhibits high genetic diversity, particularly in tropical and subtropical regions, which complicates control measures (Galiano-Carneiro and Miedaner, 2017). The disease cycle of S. turcica involves the production of conidia that are dispersed by wind and rain, leading to infection of maize leaves. The fungus can survive in crop residues, making crop rotation and residue management critical for disease control (Welz and Geiger, 2000).
2.2 Symptoms and diagnosis
The primary symptoms of NCLB include elongated, grayish-green to tan lesions on the leaves, which can coalesce to form large necrotic areas, significantly reducing photosynthetic capacity (Yang et al., 2021; Zhu et al., 2023b). These lesions are typically bordered by a chlorotic halo. In severe cases, the lesions can extend to the stalk and husks, further compromising plant health and yield (Razzaq et al., 2019; Thatcher et al., 2022). Diagnosis of NCLB is primarily based on visual inspection of these characteristic lesions. Molecular techniques, such as PCR, can also be employed to confirm the presence of S. turcica (Li et al., 2020).
2.3 Economic impact of NCLB
NCLB is one of the most economically important foliar diseases of maize, causing significant yield losses globally (Ranganatha et al., 2021). In Asia, for instance, NCLB is a major reason behind yield reductions in maize crops (Rashid et al., 2020). The economic impact is exacerbated by the need for increased fungicide applications and the potential for reduced grain quality (Rossi et al., 2022). The deployment of resistant cultivars is considered the most effective and economical method to manage NCLB, as it reduces the reliance on chemical controls and mitigates yield losses (Chen et al., 2015; Zhu et al., 2023a). Quantitative resistance, which is controlled by multiple genes, is particularly valuable as it tends to be more durable and effective across different environmental conditions (Welz and Geiger, 2000; Galiano-Carneiro and Miedaner, 2017).
3 Mechanisms of NCLB Resistance in Maize
3.1 Genetic basis of resistance
The genetic basis of resistance to northern corn leaf blight (NCLB) in maize is complex and involves both qualitative and quantitative resistance genes. Major resistance genes, such as Ht1, Ht2, Ht3, and Htn1, have been identified and mapped to specific chromosomal locations. These genes encode proteins that play crucial roles in the plant’s defense mechanisms. For instance, Ht2 and Ht3 are allelic to the wall-associated receptor-like kinase gene ZmWAK-RLK1, which is involved in pathogen recognition and signal transduction (Hurni et al., 2015; Yang et al., 2021). Quantitative trait loci (QTL) mapping has also revealed several regions in the maize genome that contribute to NCLB resistance. For example, QTLs on chromosomes 2, 5, and 8 have been identified, with qNCLB-8-2 explaining a significant portion of phenotypic variation (Chen et al., 2015; Ranganatha et al., 2021). These findings highlight the importance of both major genes and QTLs in conferring resistance to NCLB.
3.2 Types of NCLB resistance
NCLB resistance in maize can be broadly categorized into qualitative and quantitative resistance. Qualitative resistance is typically governed by major genes (Ht genes) that provide high levels of resistance but can be overcome by new pathogen races. For example, Ht1, Ht2, and Ht3 are known to confer race-specific resistance but may be less effective against diverse pathogen populations (Welz and Geiger, 2000; Yang et al., 2021). On the other hand, quantitative resistance is controlled by multiple genes, each contributing a small effect, and is generally more durable and stable across different environments. This type of resistance is often measured by traits such as lesion size, number of lesions per leaf, and disease severity (Zhu et al., 2023a; Zhu et al., 2023b). Quantitative resistance has been shown to be effective in various maize populations and is less likely to be overcome by pathogen evolution (Welz and Geiger, 2000).
3.3 Role of plant immune response in resistance
The plant immune response plays a critical role in NCLB resistance. One of the key components of this response is the wall-associated receptor-like kinase (RLK) encoded by genes such as Htn1. These RLKs are involved in the initial recognition of pathogen-associated molecular patterns (PAMPs) and the activation of downstream defense signaling pathways. For instance, the Htn1 gene encodes a receptor-like kinase that perceives pathogen elicitors and triggers immune responses, thereby delaying lesion formation and reducing disease severity (Hurni et al., 2015). Additionally, other signaling pathways, such as calcium signaling and MAPK signaling, have been implicated in NCLB resistance. Genes like CDPK21 and MKKK18, identified through bulked segregant analysis, are involved in these pathways and contribute to the plant's defense mechanisms (Li et al., 2020). The integration of these immune responses helps in mounting a robust defense against NCLB, thereby enhancing resistance in maize.
4 Gene Mining Techniques for Identifying NCLB Resistance Genes
4.1 Genome-wide association studies (GWAS)
Genome-wide association studies (GWAS) are a powerful tool for identifying genetic loci associated with traits of interest (Tam et al., 2019; Uffelmann et al., 2021; Fu, 2024), such as resistance to northern corn leaf blight (NCLB) in maize. GWAS involves scanning the genome for single nucleotide polymorphisms (SNPs) that occur more frequently in individuals with a particular trait. This method has been successfully applied to identify multiple loci associated with NCLB resistance. For instance, a high-resolution GWAS conducted on tropical maize germplasm identified 22 SNPs significantly associated with NCLB resistance, with several SNPs co-located in chromosomal bins previously reported for major resistance genes like Ht2, Ht3, and Htn1 (Rashid et al., 2020). Another study using a vast commercial maize germplasm set identified four SNP markers significantly associated with NCLB resistance, explaining up to 14.29% of the genetic variance (Inghelandt et al., 2012). These findings underscore the utility of GWAS in uncovering the genetic architecture of NCLB resistance and facilitating marker-assisted selection in breeding programs.
4.2 Quantitative trait loci (QTL) mapping
Quantitative trait loci (QTL) mapping is another essential technique for identifying regions of the genome associated with NCLB resistance. This method involves crossing two parent lines with contrasting phenotypes and analyzing the progeny to identify genomic regions linked to the trait (Collins et al., 2008; Druka et al., 2010). For example, a study mapping QTLs in a biparental population derived from a cross between resistant and susceptible lines identified three QTLs for NCLB resistance on chromosomes 2, 5, and 8, with the QTL qNCLB-8-2 explaining the highest phenotypic variation of 16.34% (Ranganatha et al., 2021). Another study using high-density SNPs identified stable QTLs on chromosome 5 (bin 5.04) that accounted for up to 20% of the phenotypic variation in NCLB resistance (Chen et al., 2015). These QTLs provide valuable targets for marker-assisted selection and breeding for enhanced NCLB resistance.
4.3 Comparative genomics and transcriptomics
Comparative genomics and transcriptomics offer insights into the genetic and molecular mechanisms underlying NCLB resistance by comparing the genomes and gene expression profiles of resistant and susceptible lines. For instance, integrating QTL mapping with transcriptome data has revealed candidate genes associated with NCLB resistance. A study combining QTL mapping and transcriptomics identified three candidate genes involved in the hypersensitive response, jasmonic acid pathway, and protein ubiquitination, which are crucial for aphid resistance in maize (Wang et al., 2023). Although this study focused on aphid resistance, similar approaches can be applied to NCLB resistance. Additionally, genome-wide nested association mapping using 1.6 million SNPs identified multiple candidate genes related to plant defense, including receptor-like kinase genes involved in basal defense, suggesting a mechanistic overlap with quantitative disease resistance (Poland et al., 2011). These integrative approaches enhance the understanding of the genetic basis of NCLB resistance and aid in the development of resistant maize varieties.
5 Key Genes and QTLs for NCLB Resistance in Maize
5.1 Ht genes
The Ht genes are a group of major resistance genes that have been extensively studied for their role in conferring resistance to northern corn leaf blight (NCLB) in maize. The primary Ht genes include Ht1, Ht2, Ht3, and Htn1. The Ht1 gene encodes a nucleotide-binding leucine-rich repeat (NLR) immune receptor. This gene was identified through map-based cloning and has been shown to confer resistance to Exserohilum turcicum race 0. Transgenic maize plants expressing the Ht1 gene exhibit a significant reduction in fungal biomass and an increase in defense-related gene expression (Thatcher et al., 2022). Ht2 and Ht3 are allelic to the Htn1 gene and encode variants of the wall-associated receptor-like kinase gene ZmWAK-RLK1. The Ht2 and Ht3 alleles differ from Htn1 by multiple amino acid polymorphisms, particularly in the extracellular domain, which affects their resistance spectra and responses (Yang et al., 2021). The Htn1 gene encodes a wall-associated receptor-like kinase (RLK) that plays a crucial role in the plant's innate immune system. It confers quantitative and partial resistance to NCLB by delaying lesion formation. The gene was originally introduced from a Mexican landrace into modern maize breeding lines (Hurni et al., 2015; Zhu et al., 2023a).
5.2 Recently identified resistance genes and QTLs
Recent studies have identified several new resistance genes and quantitative trait loci (QTLs) that contribute to NCLB resistance in maize. The QTL qNCLB7.02, located on chromosome 7, has been identified as a major contributor to NCLB resistance. It accounts for a significant portion of the phenotypic variation in disease resistance and has been validated using chromosome segment substitution lines (CSSLs) (Wang et al., 2018). Another important QTL located on chromosome 5, qNCLB5.04, has been associated with both NCLB score and lesion size. This QTL explains a substantial percentage of the phenotypic variation and is a desirable target for marker-assisted selection (Chen et al., 2015). The genes CDPK21, HEX9, and MKKK18, were identified using bulked segregant analysis (BSA) and whole genome re-sequencing. They are associated with calcium signaling, sugar signaling, and MAPK signaling pathways, respectively, and play a role in NCLB resistance (Li et al., 2020).
5.3 Functional validation of resistance genes
Functional validation of resistance genes is crucial for confirming their role in conferring resistance to NCLB. The functional role of Htn1 has been validated through TILLING mutants, which showed increased susceptibility to NCLB compared to wild-type plants. This gene encodes a wall-associated receptor-like kinase that is essential for host resistance against adapted fungal pathogens (Hurni et al., 2015). The Ht1 gene’s function was confirmed through transgenic testing, where the introduction of the native Ht1 sequence into a susceptible maize variety resulted in resistance to E. turcicum race 0. This validation highlights the gene’s role in reducing fungal biomass and enhancing defense responses (Thatcher et al., 2022). The resistance effect of qNCLB7.02 was validated using CSSLs, which demonstrated greater resistance in lines carrying this QTL compared to those without it. This validation underscores the QTL’s significance in breeding programs (Wang et al., 2018).
6 Breeding Strategies for NCLB Resistance
6.1 Traditional breeding approaches
Traditional breeding approaches for northern corn leaf blight (NCLB) resistance in maize have primarily focused on the utilization of dominant resistance genes such as Ht1, Ht2, Ht3, and Htn1. These genes have been identified as major sources of genetic resistance against the pathogen Setosphaeria turcica (Figure 1) (Hurni et al., 2015; Yang et al., 2021). The effectiveness of these genes has been demonstrated through various breeding programs, which have shown that incorporating these genes into maize cultivars can significantly reduce disease severity and improve yield under NCLB epidemic conditions. Traditional breeding also involves the use of polygenes (PG) and their combinations with dominant genes to enhance resistance levels and yield traits (Zhu et al., 2023a; Zhu et al., 2023b; Zhou and Xu, 2024). The genetic action of these combinations has been studied to predict losses and effects on yield traits, indicating that resistant genes can increase yield more efficiently under NCLB epidemic environments (Zhu et al., 2023a).
 Figure 1 Map-based cloning of Ht2/Ht3 (Adopted from Yang et al., 2021) Image caption: (a) Genetic mapping of Ht2. Diseased lesions of parental lines RP5 and RP5Ht2 are given; (b) Genetic mapping of Ht3; Diseased lesions of parental lines RP6 and RP6Ht3 are given. Genetic position of the molecular markers is given based on the position in the B73 reference genome; Only critical markers are indicated, and important flanking markers are highlighted in red. Number of tested recombinants in each recombination type is represented. Het, heterozygous; R: Rresistant; S: Susceptible; (c) Snapshot of the flanking segments genotyped using a 600K single nucleotide polymorphism array; The fragment, which is defined by two flanking markers PZE-108092843 and PZE-108096469, includes 2.81 Mb with 675 single nucleotide polymorphism markers; (d) High-resolution mapping of Ht2/Ht3; (e) Construction of the physical map surrounding Ht2/Ht3; (f) Gene structure of ZmWAK-RLK1. GUB_WAK, wall-associated receptor kinase galacturonan binding; non-RD Ser/Thr kinase, non-arginine- aspartate serine/threonine kinase domain; SP: Signal peptide; TM: Transmembrane domain; WAK: Wall-associated kinase domain (Adopted from Yang et al., 2021) |
6.2 Marker-assisted selection (MAS) for NCLB resistance
Marker-assisted selection (MAS) has become a pivotal strategy in breeding for NCLB resistance. This approach leverages molecular markers linked to resistance genes to facilitate the selection process (Francia et al., 2005; Hasan et al., 2021). For instance, the identification of quantitative trait loci (QTL) such as qNCLB5.04 and qNCLB7.02, which confer significant resistance to NCLB, has been instrumental in MAS. These QTLs have been mapped using high-density SNP markers, allowing for precise selection of resistant genotypes (Chen et al., 2015; Wang et al., 2018). Additionally, bulked segregant analysis (BSA) combined with whole genome re-sequencing has identified key candidate genes like CDPK21, HEX9, and MKKK18, which are associated with NCLB resistance and can be targeted in MAS programs (Li et al., 2020). The integration of these molecular markers into breeding programs has enhanced the efficiency and accuracy of developing NCLB-resistant maize varieties.
6.3 Genomic selection and precision breeding
Genomic selection (GS) and precision breeding represent advanced strategies for improving NCLB resistance in maize. GS involves the use of genome-wide markers to predict the breeding values of individuals, thereby accelerating the selection process (Crossa e#ret al., 2017; Liang, 2024). Studies have shown that combining training sets from different heterotic groups can significantly increase prediction accuracies for NCLB resistance (Technow et al., 2013). This approach allows for the selection of genotypes with superior resistance traits without the need for extensive phenotyping. Furthermore, genomics-assisted breeding, which integrates genomic data with traditional breeding methods, has been proposed as a way to enhance resistance to multiple diseases, including NCLB. This method involves the use of genomic models to predict the performance of untested genotypes, thereby facilitating the introgression of resistance genes from diverse genetic backgrounds into elite breeding lines (Figure 2) (Miedaner et al., 2020). The application of these advanced breeding strategies holds great promise for developing maize cultivars with durable and broad-spectrum resistance to NCLB.
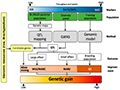 Figure 2 Techniques for genomics-assisted breeding (Adopted from Miedaner et al., 2020) Image caption: SSR=Single sequence repeat, DArT=Diversity array technique, SNP=Single nucleotide polymorphism, NGS=Next-generation sequencing, QTL=Quantitative trait locus, GWAS=Genome-wide association studies, MAS=Marker-assisted selection, MABC=Marker-assisted backcrossing, MARS=Marker-assisted recurrent selection, GS=Genomic selection (Adopted from Miedaner et al., 2020) |
7 Biotechnological Approaches for Enhancing NCLB Resistance
7.1 CRISPR/Cas9 and gene editing
CRISPR/Cas9 technology has revolutionized the field of genetic engineering by enabling precise modifications to the genome. This method can be employed to enhance resistance to northern corn leaf blight (NCLB) in maize by targeting and editing specific genes associated with disease resistance. For instance, the Ht1, Ht2, Ht3, and Htn1 genes, which are major sources of genetic resistance against NCLB, can be edited to improve their efficacy or to combine multiple resistance traits into a single cultivar (Hurni et al., 2015; Wang et al., 2018; Yang et al., 2021). The use of CRISPR/Cas9 allows for the introduction of beneficial alleles or the removal of susceptibility genes, thereby creating maize varieties with enhanced resistance to NCLB.
7.2 RNA interference (RNAi) and gene silencing
RNA interference (RNAi) is another powerful tool for enhancing NCLB resistance in maize. This technique involves the silencing of specific genes that are crucial for the pathogen’s ability to infect the host plant. By designing RNAi constructs that target essential genes of the fungal pathogen Setosphaeria turcica, it is possible to reduce the pathogen's virulence and thereby increase the plant's resistance to NCLB. Studies have shown that RNAi can be effectively used to silence genes involved in the pathogen’s infection process, leading to a significant reduction in disease severity (Welz and Geiger, 2000; Li et al., 2020). This approach can be combined with traditional breeding methods to develop maize varieties with durable resistance to NCLB.
7.3 Transgenic approaches
Transgenic approaches involve the introduction of foreign genes into the maize genome to confer resistance to NCLB. This can include the insertion of genes encoding for pathogenesis-related proteins, receptor-like kinases, or other defense-related proteins. For example, the Htn1 gene, which encodes a wall-associated receptor-like kinase, has been shown to confer quantitative resistance to NCLB by delaying lesion formation and reducing disease severity (Hurni et al., 2015; Yang et al., 2021). Transgenic maize lines expressing Htn1 or other resistance genes can be developed to provide robust protection against NCLB. Additionally, the use of transgenic approaches allows for the stacking of multiple resistance genes, thereby enhancing the overall resistance of the maize plant to various strains of the pathogen (Chen et al., 2015; Rashid et al., 2020; Ranganatha et al., 2021).
8 Case Study: Successful Breeding Program for NCLB-Resistant Maize
8.1 Overview of the breeding program
The breeding program aimed at developing northern corn leaf blight (NCLB)-resistant maize was initiated in response to the significant yield losses caused by the disease, particularly in tropical and subtropical regions. The program utilized a combination of genome-wide association studies (GWAS), quantitative trait loci (QTL) mapping, and marker-assisted selection (MAS) to identify and incorporate resistance genes into elite maize lines. The breeding efforts focused on both qualitative resistance genes (Ht genes) and quantitative resistance loci to ensure durable and broad-spectrum resistance.
8.2 Key techniques and findings
The program employed several advanced techniques to achieve its goals. High-resolution GWAS identified 22 significant SNPs associated with NCLB resistance across three tropical maize panels. Haplotype regression analysis further revealed 17 significant haplotypes, with some co-located with known resistance genes like Ht2, Ht3, and Htn1 (Rashid et al., 2020). Multiple QTLs for NCLB resistance were identified on various chromosomes. Notably, qNCLB7.02 on chromosome 7 and qNCLB5.04 on chromosome 5 were found to have significant effects on resistance, explaining up to 15.29% and 20% of phenotypic variation, respectively (Figure 3) (Chen et al., 2015; Wang et al., 2018; Ranganatha et al., 2021). MAS was employed to incorporate major QTLs and resistance genes into breeding lines. This approach facilitated the development of inbred lines with combined resistance to multiple foliar diseases, including NCLB, sorghum downy mildew, and southern corn rust (Ranganatha et al., 2021). The Htn1 gene, encoding a wall-associated receptor-like kinase, was cloned and shown to confer quantitative resistance by delaying lesion formation. This gene was originally introduced from a Mexican landrace and has been a cornerstone of NCLB resistance breeding (Hurni et al., 2015).
 Figure 3 Linkage map and position of the QTL associated with northern corn leaf blight resistance mapped from F2:3 mapping population of the cross CML153×SKV50 (Adopted from Ranganatha et al., 2021) |
8.3 Implications and lessons learned
The successful breeding program for NCLB-resistant maize has several important implications and lessons. Combining qualitative and quantitative resistance genes provides a more robust defense against NCLB. The identification of allelic relationships among major resistance genes (e.g., Ht2, Ht3, and Htn1) underscores the importance of understanding genetic interactions (Hurni et al., 2015; Yang et al., 2021). The application of GWAS, QTL mapping, and MAS has significantly accelerated the breeding process. These tools enable precise identification and incorporation of resistance genes, leading to the development of high-yielding, disease-resistant varieties (Chen et al., 2015; Rashid et al., 2020; Ranganatha et al., 2021). The deployment of resistant cultivars is a sustainable and cost-effective strategy for managing NCLB. The program's success highlights the potential of genetic resistance as a cornerstone of integrated disease management in maize (Welz and Geiger, 2000; Zhu et al., 2023a; Zhu et al., 2023b). Ongoing research is essential to address the evolving nature of NCLB pathogens. The breeding program's adaptability in incorporating new resistance genes and QTLs ensures continued effectiveness against emerging pathogen races (Welz and Geiger, 2000; Li et al., 2020).
9 Challenges in NCLB Resistance Breeding
9.1 Genetic complexity and environmental interactions
Breeding for resistance to northern corn leaf blight (NCLB) in maize is complicated by the genetic complexity of the trait and its interaction with environmental factors. The resistance to NCLB is often controlled by multiple genes, including both major resistance genes (Ht genes) and quantitative trait loci (QTL) (Chen et al., 2015; Ranganatha et al., 2021; Zhu et al., 2023b). For instance, the Ht1, Ht2, Ht3, and Htn1 genes are known to confer resistance, but their effectiveness can vary depending on the environmental conditions and the genetic background of the maize lines (Hurni et al., 2015; Yang et al., 2021). Additionally, the expression of quantitative resistance is influenced by environmental factors, making it challenging to achieve stable resistance across different growing conditions (Welz and Geiger, 2000). This complexity necessitates the use of advanced breeding techniques such as genome-wide association studies (GWAS) and marker-assisted selection to identify and combine multiple resistance alleles (Ding et al., 2015; Rashid et al., 2020).
9.2 Evolution of pathogen virulence
The evolution of pathogen virulence poses a significant challenge to NCLB resistance breeding. The fungal pathogen Setosphaeria turcica, which causes NCLB, can rapidly evolve new virulent races that overcome existing resistance genes (Welz and Geiger, 2000; Yang et al., 2021). For example, while the Ht genes provide resistance to specific races of the pathogen, new races can emerge that are capable of infecting previously resistant maize varieties (Hurni et al., 2015). This dynamic interaction between the host and the pathogen requires continuous monitoring and the development of new resistance genes to stay ahead of the evolving pathogen (Li et al., 2020). The identification of novel resistance genes and the pyramiding of multiple resistance genes are essential strategies to enhance the durability of NCLB resistance (Chen et al., 2015; Rashid et al., 2020).
9.3 Regulatory and market constraints
Regulatory and market constraints also impact the breeding and deployment of NCLB-resistant maize varieties. The development and release of new resistant varieties must comply with regulatory requirements, which can be time-consuming and costly (Zhu et al., 2023b). Additionally, market acceptance of new varieties depends on their agronomic performance, including yield and other desirable traits, in addition to disease resistance (Zhu et al., 2023a). Breeders must balance the need for NCLB resistance with other important traits to ensure that new varieties meet the demands of farmers and consumers (Ding et al., 2015; Zhu et al., 2023a). Furthermore, the adoption of resistant varieties can be influenced by the availability of seeds, the cost of new varieties, and the willingness of farmers to switch from traditional varieties (Welz and Geiger, 2000). These factors highlight the importance of a holistic approach to breeding that considers both biological and socio-economic aspects.
10 Suggestion and Prospect
The study of gene mining and breeding strategies for northern corn leaf blight (NCLB) resistance in maize revealed several key findings. The identification and characterization of resistance genes (R-genes) have significantly advanced, with a focus on quantitative trait loci (QTLs) contributing to partial resistance across various maize genotypes. Effective utilization of these R-genes requires their integration into breeding programs through marker-assisted selection (MAS) and genomic selection (GS). Breeding strategies that combine conventional methods with molecular tools have demonstrated potential to improve resistance, particularly by enhancing genetic diversity and pyramiding multiple resistance genes. Moreover, advancements in genome editing techniques, such as CRISPR-Cas9, have opened up new avenues for targeted gene modification, offering precise manipulation of resistance-associated genes to strengthen NCLB resistance.
Future research should focus on several key areas. Conduct comprehensive functional analyses of known and newly discovered resistance genes to gain deeper insights into their roles in resistance against northern corn leaf blight (NCLB), elucidating specific mechanisms of pathogen recognition and host response. By employing genomic selection strategies, the breeding process for resistant varieties can be accelerated, leveraging genome-wide marker data and predictive models to improve selection efficiency. Expanding the genetic base is also crucial, which can be achieved by incorporating underutilized germplasm and wild maize relatives. These genetic resources may harbor untapped resistance genes, offering support for broader defense mechanisms. Focus on gene pyramiding to stack multiple resistance genes, achieving more durable and robust resistance, thereby reducing the risk of pathogen breakthroughs against single-gene resistance. Further optimize and integrate CRISPR-related tools to precisely edit or modulate the expression of resistance genes, ensuring enhanced disease resistance without compromising agronomic traits.
Besides, conduct trials across different geographical and climatic regions to evaluate the stability and durability of resistance in diverse environments, which is essential for the global deployment of resistant cultivars. Consider holistic breeding strategies that incorporate resistance alongside yield, drought tolerance, and other agronomic traits to enhance the adoption of resistant varieties. Establish robust pathogen monitoring systems to track the evolution of Exserohilum turcicum, the causal agent of NCLB, ensuring resistance strategies remain effective against emerging virulent strains. Utilize bioinformatics tools to integrate genomic, transcriptomic, and proteomic data, building comprehensive models of resistance pathways that can guide targeted breeding strategies. Foster collaborative networks among researchers, breeders, farmers, and policymakers to ensure knowledge exchange and align breeding goals with practical agricultural needs.
Achieving durable resistance to northern corn leaf blight in maize is a complex challenge requiring a multifaceted approach that blends traditional breeding, modern molecular tools, and strategic field management. By building upon foundational genetic knowledge and fostering innovation in breeding methodologies, stakeholders can collectively advance toward cultivars that offer sustainable, long-term resistance. This integration of genetic insights with practical breeding applications holds the promise of not only mitigating the impact of NCLB but also bolstering global maize productivity and food security.
Acknowledgments
Thank you to the anonymous peer review for providing targeted revision suggestions for the manuscript.
Funding
This research was fiinded by agrant from Science and Technology Innovation and Demonstration Promotion Fund Project of Hangzhou Academy of Agricultural Sciences (2022HNCT-08).
Conflict of Interest Disclosure
The authors affirm that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Chen G.S., Wang X.M., Long S.S., Jaqueth J., Li B.L., Yan J.B., and Ding J.Q., 2015, Mapping of QTL conferring resistance to northern corn leaf blight using high-density SNPs in maize, Molecular Breeding, 36: 1-9.
Collins N.C., Tardieu F., and Tuberosa R., 2008, Quantitative trait loci and crop performance under abiotic stress: where do we stand? Plant Physiology, 147(2): 469-486.
https://doi.org/10.1104/pp.108.118117
PMID: 18524878 PMCID: PMC2409033
Crossa J., Pérez-Rodríguez P., Cuevas J., Montesinos-López O., Jarquín D., de los Campos G., Burgueño J., González-Camacho J.M., Pérez-Elizalde S., Beyene Y., Dreisigacker S., Singh R., Zhang X., Gowda M., Roorkiwal M., Rutkoski J., and Varshney R.K., 2017, Genomic selection in plant breeding: methods, models, and perspectives, Trends in Plant Science, 22(11): 961-975.
https://doi.org/10.1016/j.tplants.2017.08.011
PMID: 28965742
Ding J.Q., Ali F., Chen G.A., Li H.H., Mahuku G., Yang N., Narro L., Magorokosho C., Makumbi D., and Yan J.B., 2015, Genome-wide association mapping reveals novel sources of resistance to northern corn leaf blight in maize, BMC Plant Biology, 15: 206.
https://doi.org/10.1186/s12870-015-0589-z
PMID: 26289207 PMCID: PMC4546088
Druka A., Potokina E., Luo Z.W., Jiang N., Chen X.W., Kearsey M., and Waugh R., 2010, Expression quantitative trait loci analysis in plants, Plant Biotechnology Journal, 8(1): 10-27.
https://doi.org/10.1111/j.1467-7652.2009.00460.x
De Rossi R.L.D., Guerra F.A., Plazas M.C., Vuletic E.E., Brücher E., Guerra G.D., and Reis E.M., 2022, Crop damage, economic losses, and the economic damage threshold for northern corn leaf blight, Crop Protection, 154: 105901.
https://doi.org/10.1016/j.cropro.2021.105901
Francia E., Tacconi G., Crosatti C., Barabaschi D., Bulgarelli D., Dall’Aglio E., and Valè G., 2005, Marker assisted selection in crop plants, Plant Cell, Tissue and Organ Culture, 82: 317-342.
https://doi.org/10.1007/s11240-005-2387-z
Fu C., 2024, Application of genome-wide association study in crop disease resistance breeding, Field Crop, 7(1): 1-8.
https://doi.org/10.5376/fc.2024.07.0001
Galiano-Carneiro A., and Miedaner T., 2017, Genetics of resistance and pathogenicity in the maize/Setosphaeria turcica pathosystem and implications for breeding, Frontiers in Plant Science, 8: 1490.
https://doi.org/10.3389/fpls.2017.01490
PMID: 28900437 PMCID: PMC5581881
Hasan N., Choudhary S., Naaz N., Sharma N., and Laskar R.A., 2021, Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes, Journal of Genetic Engineering and Biotechnology, 19(1): 128.
https://doi.org/10.1186/s43141-021-00231-1
PMID: 34448979 PMCID: PMC8397809
Hurni S., Scheuermann D., Krattinger S., Kessel B., Wicker T., Herren G., Fitze M., Breen J., Presterl T., Ouzunova M., and Keller B., 2015, The maize disease resistance gene Htn1 against northern corn leaf blight encodes a wall-associated receptor-like kinase, Proceedings of the National Academy of Sciences, 112(28): 8780-8785.
https://doi.org/10.1073/pnas.1502522112
PMID: 26124097 PMCID: PMC4507197
Li C.L., Ling F.L., Su G.H., Sun W.F., Liu H.S., Su Y.C., and Qi X., 2020, Location and mapping of the NCLB resistance genes in maize by bulked segregant analysis (BSA) using whole genome re-sequencing, Molecular Breeding, 40: 1-12.
https://doi.org/10.1007/s11032-020-01171-3
Liang K.W., 2024, Implementing genomic selection in sugarcane breeding programs: challenges and opportunities, Plant Gene and Trait, 15(1): 23-32.
https://doi.org/10.5376/pgt.2024.15.0004
Miedaner T., Boeven A.L.G.C., Gaikpa D.S., Kistner M.B., and Grote C.P., 2020, Genomics-assisted breeding for quantitative disease resistances in small-grain cereals and maize, International Journal of Molecular Sciences, 21(24): 9717.
https://doi.org/10.3390/ijms21249717
PMID: 33352763 PMCID: PMC7766114
Pan S.Q., Qiao J.F., Wang R., Yu H.L., Wang C., Taylor K., and Pan H.Y., 2022, Intelligent diagnosis of northern corn leaf blight with deep learning model, Journal of Integrative Agriculture, 21(4): 1094-1105.
https://doi.org/10.1016/S2095-3119(21)63707-3
Poland J.A., Bradbury P.J., Buckler E.S., and Nelson R.J., 2011, Genome-wide nested association mapping of quantitative resistance to northern leaf blight in maize, Proceedings of the National Academy of Sciences, 108(17): 6893-6898.
https://doi.org/10.1073/pnas.1010894108
PMID: 21482771 PMCID: PMC3084105
Ranganatha H.M., Lohithaswa H.C., and Pandravada A., 2021, Mapping and validation of major quantitative trait loci for resistance to northern corn leaf blight along with the determination of the relationship between resistances to multiple foliar pathogens of maize (Zea mays L.), Frontiers in Genetics, 11: 548407.
https://doi.org/10.3389/fgene.2020.548407
PMID: 33584784 PMCID: PMC7878677
Rashid Z., Sofi M., Harlapur S.I., Kachapur R.M., Dar Z.A., Singh P.K., Zaidi P.H., Vivek B.S., and Nair S.K., 2020, Genome-wide association studies in tropical maize germplasm reveal novel and known genomic regions for resistance to northern corn leaf blight, Scientific Reports, 10(1): 21949.
https://doi.org/10.1038/s41598-020-78928-5
PMID: 33319847 PMCID: PMC7738672
Razzaq T., Khan M.F., and Awan S.I., 2019, Study of northern corn leaf blight (NCLB) on maize (Zea mays L.) genotypes and its effect on yield, Sarhad Journal of Agriculture, 35(4): 1166-1174.
http://dx.doi.org/10.17582/journal.sja/2019/35.4.1166.1174
Tam V., Patel N., Turcotte M., Bossé Y., Paré G., and Meyre D., 2019, Benefits and limitations of genome-wide association studies, Nature Reviews Genetics, 20(8): 467-484.
https://doi.org/10.1038/s41576-019-0127-1
PMID: 31068683
Technow F., Bürger A., and Melchinger A.E., 2013, Genomic prediction of northern corn leaf blight resistance in maize with combined or separated training sets for heterotic groups, G3: Genes, Genomes, Genetics, 3: 197-203.
https://doi.org/10.1534/g3.112.004630
PMID: 23390596 PMCID: PMC3564980
Thatcher S., Leonard A., Lauer M., Panangipalli G., Norman B., Hou Z.L., Llaca V., Hu W.N., Qi X.L., Jaqueth J., Severns D., Whitaker D., Wilson B., Tabor G., and Li B.L., 2022, The northern corn leaf blight resistance gene Ht1 encodes an nucleotide‐binding, leucine‐rich repeat immune receptor, Molecular Plant Pathology, 24(7): 758-767.
https://doi.org/10.1111/mpp.13267
PMID: 36180934 PMCID: PMC10257041
Uffelmann E., Huang Q.Q., Munung N.S., de Vries J., Okada Y., Martin A.R., Martin H.C., Lappalainen T., and Posthuma D., 2021, Genome-wide association studies, Nature Reviews Methods Primers, 1(1): 59.
https://doi.org/10.1038/s43586-021-00056-9
van Inghelandt D., Melchinger A.E, Martinant J.P., and Stich B., 2012, Genome-wide association mapping of flowering time and northern corn leaf blight (Setosphaeria turcica) resistance in a vast commercial maize germplasm set, BMC Plant Biology, 12: 56.
https://doi.org/10.1186/1471-2229-12-56
PMID: 22545925 PMCID: PMC3511189
Wang J.J., Xu Z.N., Yang J., Lu X.H., Zhou Z.S., Zhang C.S., Zheng L., Tian R., Hao Z.F., Yong H.J., Li M.S., Zhang D.G., Li X.H., and Weng J.F., 2018, qNCLB7.02, a novel QTL for resistance to northern corn leaf blight in maize, Molecular Breeding, 38: 1-2.
https://doi.org/10.1007/s11032-017-0770-1
Wang T.Y., Wang K.J., Wang C.H., Zhao Y.B., Tao Z., Li J.Y., Wang L., Shi J., Huang S.J., Xie C.X., and Li P.J., 2023, Combining QTL mapping with multiomics profiling reveals genetic control of corn leaf aphid (Rhopalosiphum maidis) resistance in maize, Journal of Experimental Botany, 74(12): 3749-3764.
https://doi.org/10.1093/jxb/erad113
PMID: 36964900
Welz H.G., and Geiger H.H., 2000, Genes for resistance to northern corn leaf blight in diverse maize populations, Plant Breeding, 119(1): 1-14.
https://doi.org/10.1046/J.1439-0523.2000.00462.X
Yang P., Scheuermann D., Kessel B., Koller T., Greenwood J.R., Hurni S., Herren G., Zhou S.H., Marande W., Wicker T., Krattinger S.G., Ouzunova M., and Keller B., 2021, Alleles of a wall-associated kinase gene account for three of the major northern corn leaf blight resistance loci in maize, The Plant Journal, 106(2): 526-535.
https://doi.org/10.1111/tpj.15183
PMID: 33533097
Zhu X.Y., Kebede A., Woldemariam T., Wu J.H., Jindal K.K., and Reid L.M., 2023a, Resistance breeding to northern corn leaf blight with dominant genes, polygene, and their combinations-effects to yield traits, Agronomy, 13(5): 1269.
https://doi.org/10.3390/agronomy13051269
Zhu X.Y., Reid L.M., Woldemariam T., Wu J.H., Jindal K.K., and Kebede A., 2023b, Resistance breeding for northern corn leaf blight with dominant genes, polygene, and their combinations-effects on disease traits, Agronomy, 13(4): 1096.
https://doi.org/10.3390/agronomy13041096

. PDF(0KB)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Lin Zhao
. Yifan Wu
. Xiangqun Yu
. Jiang Shi
Related articles
. Northern corn leaf blight
. Maize
. Resistance breeding
. Gene mining
. Marker-assisted selection
Tools
. Email to a friend
. Post a comment


